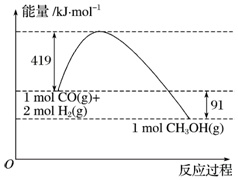
甲醇是重要的工业原料.煤化工可以利用煤炭制取水煤气从而合成甲醇:CO(g)+2H2(g)⇌CH3OH(g). 化学键 H-H H-O O=O 键能kJ/mol 436 x 496 已知:①图为反应的能量变化和物质键能②CO(g)+ 1 2 O2(g)═CO2(g)△H=-280kJ/molH2(g)+
2019-11-27
甲醇是重要的工业原料.煤化工可以利用煤炭制取水煤气从而合成甲醇:CO(g)+2H2(g)⇌CH3OH(g).
已知:
①图为反应的能量变化和物质键能
②CO(g)+
O2(g)═CO2(g)△H=-280kJ/mol
H2(g)+
O2(g)═H2O(l)△H=-284kJ/mol
H2O(l)═H2O(g)△H=+44kJ/mol
请回答下列问题:
(1)请写出表示气态甲醇燃烧热的热化学方程式___.
(2)H-O键的键能x为___kJ/mol.
(3)甲醇气体分解为CO和H2两种气体的反应的活化能为___kJ/mol.
| 化学键 | H-H | H-O | O=O |
| 键能kJ/mol | 436 | x | 496 |
①图为反应的能量变化和物质键能
②CO(g)+
| 1 |
| 2 |
H2(g)+
| 1 |
| 2 |
H2O(l)═H2O(g)△H=+44kJ/mol
请回答下列问题:

(1)请写出表示气态甲醇燃烧热的热化学方程式___.
(2)H-O键的键能x为___kJ/mol.
(3)甲醇气体分解为CO和H2两种气体的反应的活化能为___kJ/mol.