(2012•茂名一模)甲醇应用于精细化工,塑料等领域,是基础的有机化工原料和优质燃料.工业上利用H2、CO和CO2等气体体系在催化剂条件下合成甲醇.主要反应:①CO(g)+2H2(g)⇌CH3OH(g)△H=-91kJ•mol-1②CO2(g)+3H2(g)⇌CH3OH(g)+H2O(g)△H=-49kJ•mol-1副反应:2CO(g)+4H2(g)⇌CH3OCH3(g)+H2O(g)△H=-206kJ•mol-1(1)写出反应①平衡常数的表达式K=c(CH3OH)c(CO)•c2(H2)K=c(CH3O
2019-11-23
(2012•茂名一模)甲醇应用于精细化工,塑料等领域,是基础的有机化工原料和优质燃料.工业上利用H2、CO和CO2等气体体系在催化剂条件下合成甲醇.
主要反应:①CO(g)+2H2(g)⇌CH3OH(g)△H=-91kJ•mol-1
②CO2(g)+3H2(g)⇌CH3OH(g)+H2O(g)△H=-49kJ•mol-1
副反应:2CO(g)+4H2(g)⇌CH3OCH3(g)+H2O(g)△H=-206kJ•mol-1
(1)写出反应①平衡常数的表达式
(2)工业上可利用CH3OH(g)制备CH3OCH3(g),写出热化学方程式______.
(3)生产中H2和CO按物质的量比为10:1投料,假设在反应容器中投入1mol CO和10mol H2在某温度下反应(只考虑反应①),达到反应限度后,测得甲醇的物质的量分数为5%,计算CO转化率,写出计算过程.______
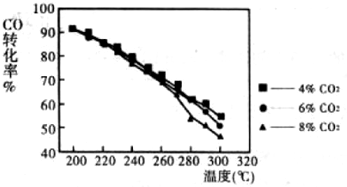
(4)为寻找原料气中合适的CO2浓度,进行不同的实验,每次实验的压强及H2和CO投料比(物质的量比为10:1)相同,实验情况如图,CO2浓度对CO转化率影响规律是:______,其原因是______.
(5)工业中常用甲醇为燃料制成燃料电池,请写出在氢氧化钾介质中该电池的负极反应式______.
主要反应:①CO(g)+2H2(g)⇌CH3OH(g)△H=-91kJ•mol-1
②CO2(g)+3H2(g)⇌CH3OH(g)+H2O(g)△H=-49kJ•mol-1
副反应:2CO(g)+4H2(g)⇌CH3OCH3(g)+H2O(g)△H=-206kJ•mol-1
(1)写出反应①平衡常数的表达式
K=
| c(CH3OH) |
| c(CO)•c2(H2) |
K=
.| c(CH3OH) |
| c(CO)•c2(H2) |
(2)工业上可利用CH3OH(g)制备CH3OCH3(g),写出热化学方程式______.
(3)生产中H2和CO按物质的量比为10:1投料,假设在反应容器中投入1mol CO和10mol H2在某温度下反应(只考虑反应①),达到反应限度后,测得甲醇的物质的量分数为5%,计算CO转化率,写出计算过程.______
(4)为寻找原料气中合适的CO2浓度,进行不同的实验,每次实验的压强及H2和CO投料比(物质的量比为10:1)相同,实验情况如图,CO2浓度对CO转化率影响规律是:______,其原因是______.

(5)工业中常用甲醇为燃料制成燃料电池,请写出在氢氧化钾介质中该电池的负极反应式______.